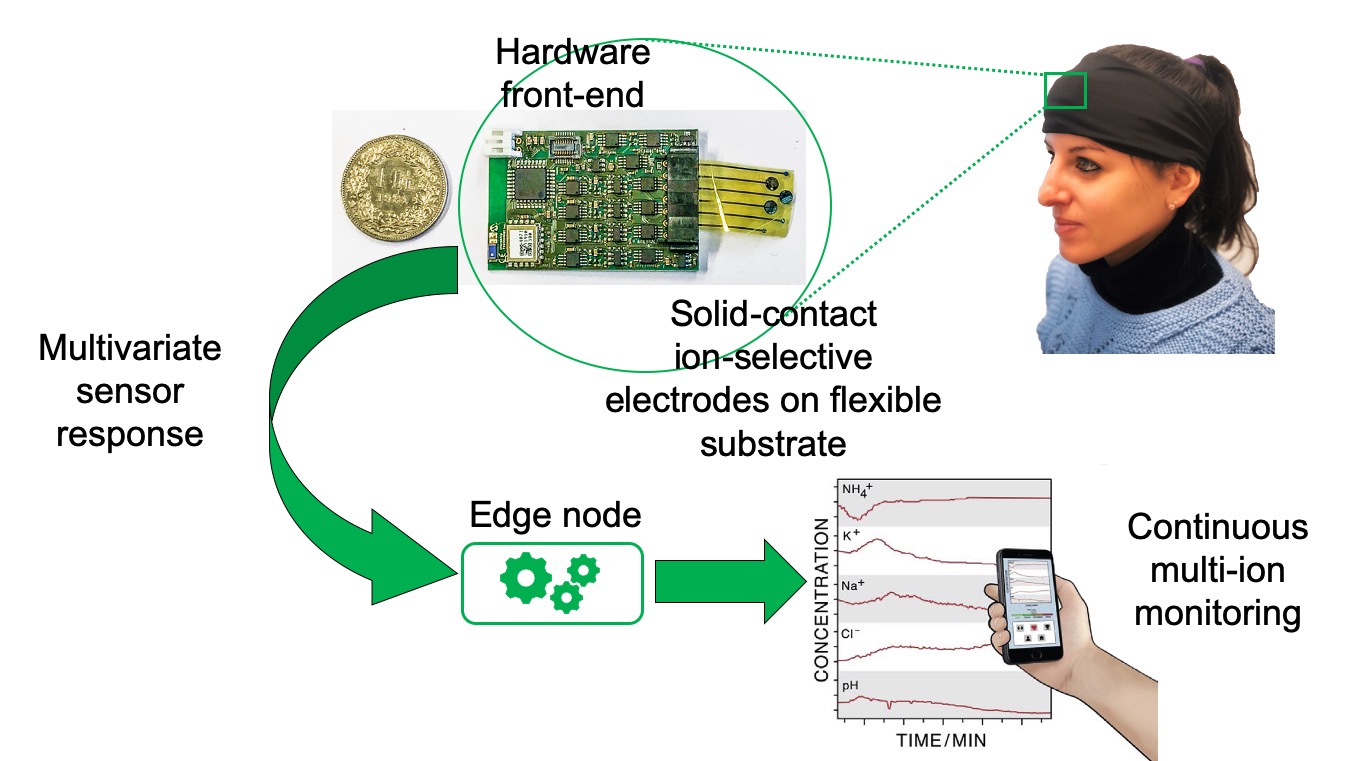
System overview
With the growth of personalized medicine and e-Health, there has been an increasing interest in the development of accurate sensing systems able to support this healthcare revolution for both in- and off-hospital monitoring. Wearable devices occupy a primary role in this data-driven revolution to collect data in medicine. Despite the great research efforts, current wearable biosensors still have several issues, such as poor collection, separate sampling and analysis, low multi-sensing capabilities, and lack of data correlating sweat and blood values. Progress in materials science to enhance sensitivity, selectivity, detection range, and reduce costs is also needed.
We investigate and address some of the challenges related to wearable chemical sensors, with particular interest on sweat sensing. This field is very attractive as this biofluid is highly promising as alternative to blood in diagnostics. In fact, it is readily accessible and reproducible, it does not require invasive procedures like blood collection, it contains several recognized biomarkers with diagnostic capabilities and good correlation with blood values, and it can be easily monitored continuously. A wearable electrochemical platform addressing some of these challenges is fabricated. This device enables the monitoring of several ions for different applications: Li+ for Therapeutic Drug Monitoring (TDM) of people affected by psychiatric disorders, Pb2+ for the control of heavy metal contamination, K+ and Na+ for tracking of physical exercise.

System overview
The sensing is based on the use of Solid-Contact Ion-Selective Electrodes (SC-ISEs) with noble metal nanostructures. These materials are efficiently deposited in a fast on-step electrodeposition process (less than 4 minutes). We demonstrate the superior behavior of the sensors in terms of sensitivity, selectivity and stability, with capacitance values as high as 20 μF/mm2 and extremely low potential drift (3±2 μF/s), both on macro and miniaturized electrodes. The sensors are successfully proposed for the first time for the monitoring of lithium levels in sweat for applications in TDM of psychiatric disorders, with Nernstian response (58±2 mV/decade) in artificial perspiration and LOD of 1.4 mM (that is below the range of clinical interest of the drug in sweat 2.4-4.5 mM). Lead sensors are also fabricated and tested in sweat to propose an innovative method for the control of heavy metal contamination with good analytical parameters. In addition, the detection of sweat potassium and sodium for physical exercise and hydration monitoring is also demonstrated. A custom-made flexible electrochemical sensing system including a temperature sensor, a stable reference electrode and several ion-sensing channels is fabricated with lithographic techniques. The platform is integrated with a low-cost fluidics that enables the collection of fresh sweat and the disposal of the already-tested sample fluid. With its high accuracy, selectivity and stability, this device represents an important step towards the development of efficient and low-cost non-invasive healthcare tracking systems for e-Health.
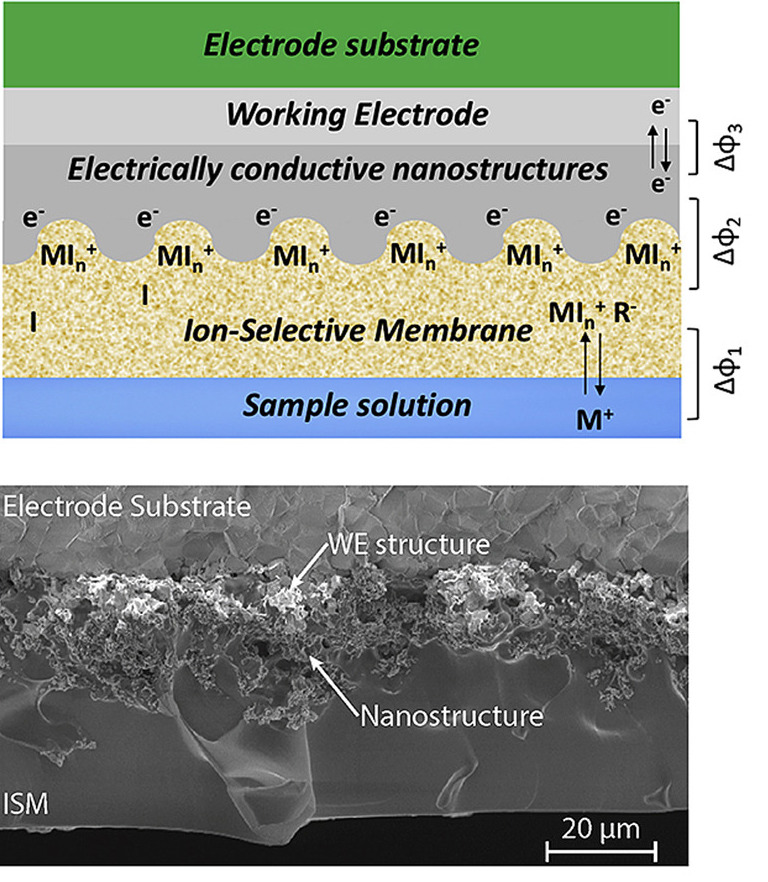

Nanostructured electrode and flexible platform
A co-design of hardware and software interfaces for multi-ion-sensors is needed to ensure an accurate, continuous, and real-time monitoring of relevant biomarkers in wearable physiology and advanced healthcare diagnosis.
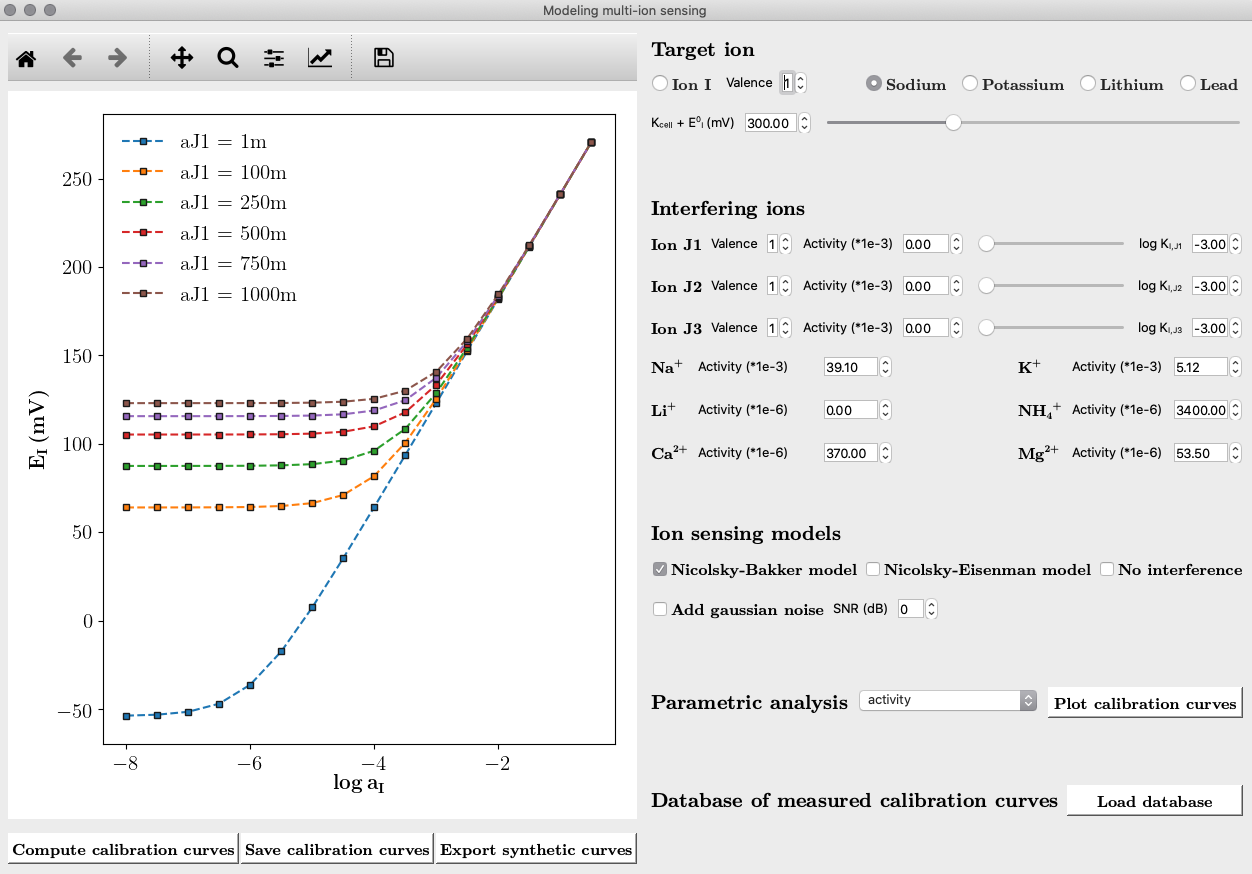
Graphic user interface
An important challenge in multi-ion-sensing arises from ion interference due to electrolytes inherently present in the biological sample, and to intrinsic sensor cross-selectivity bounds. This artifact strongly distorts sensor response and degrades limit of detection. Therefore, a compact analytical model of ion-sensing transduction mechanism through polymeric SC-ISEs is developed. This study provides both quantitative and qualitative understanding of the impact of ion interference in sensor responses. The analytical model is implemented at the core of an emulator of synthetic datasets that is built to simulate the response of polymeric ISEs in complex samples of different electrolytic composition.
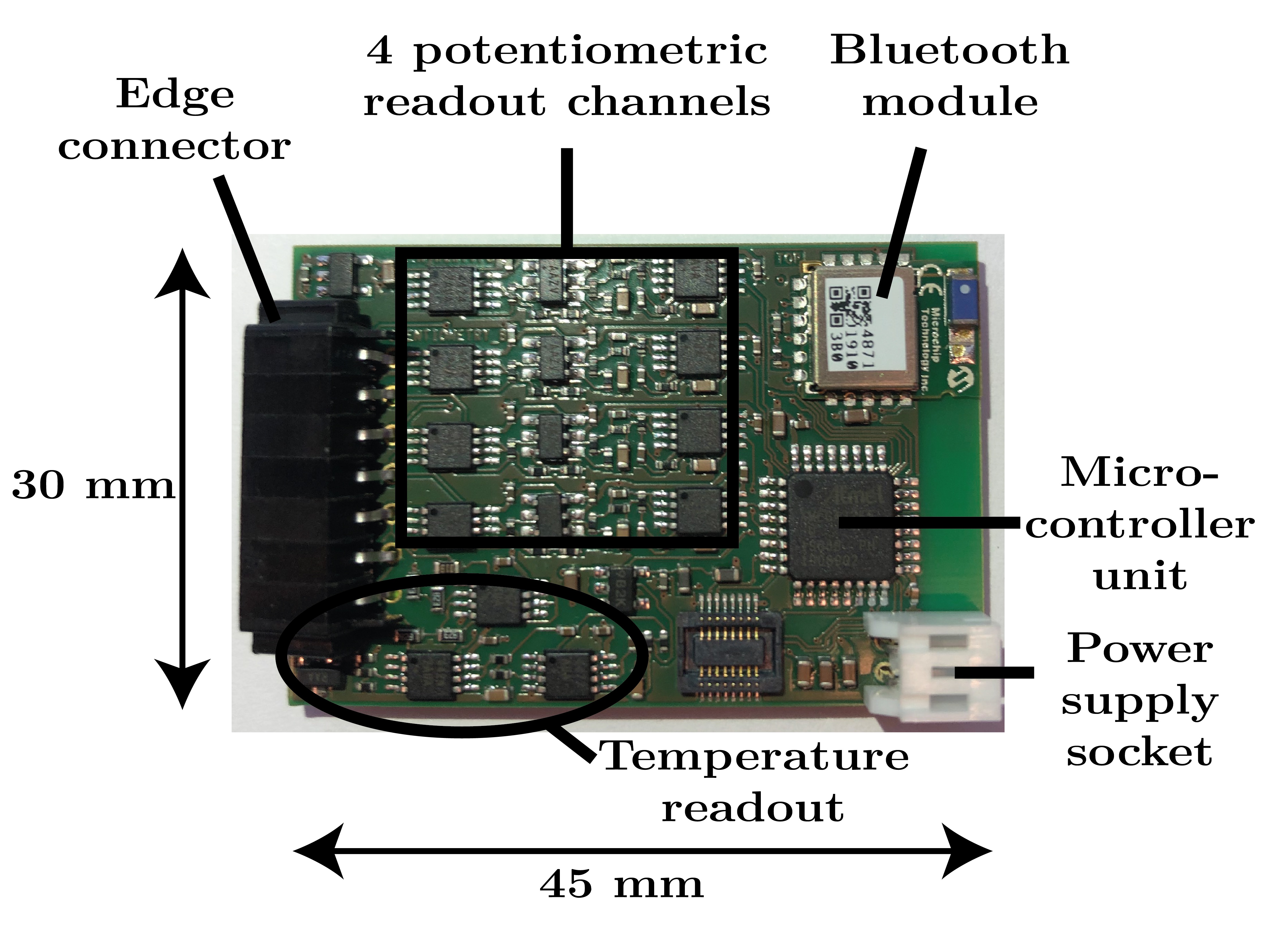
A multi-channel ion sensing front-end is designed to monitor up to four endogenous electrolytes. The hardware comprises separate potentiometric readout circuits consisting of buffered and differential circuitry. A resistive thermal device readout circuit is included to monitor in-situ temperature. Namely, an improved Howland current pump with high output impedance polarizes the temperature sensor with a DC current, and the voltage drop across the device is sensed. A Bluetooth low energy module enables remote control, data collection, and real-time feedback of the biological information. The sensing front-end is characterized through sodium, potassium, ammonium, and calcium ions monitoring.
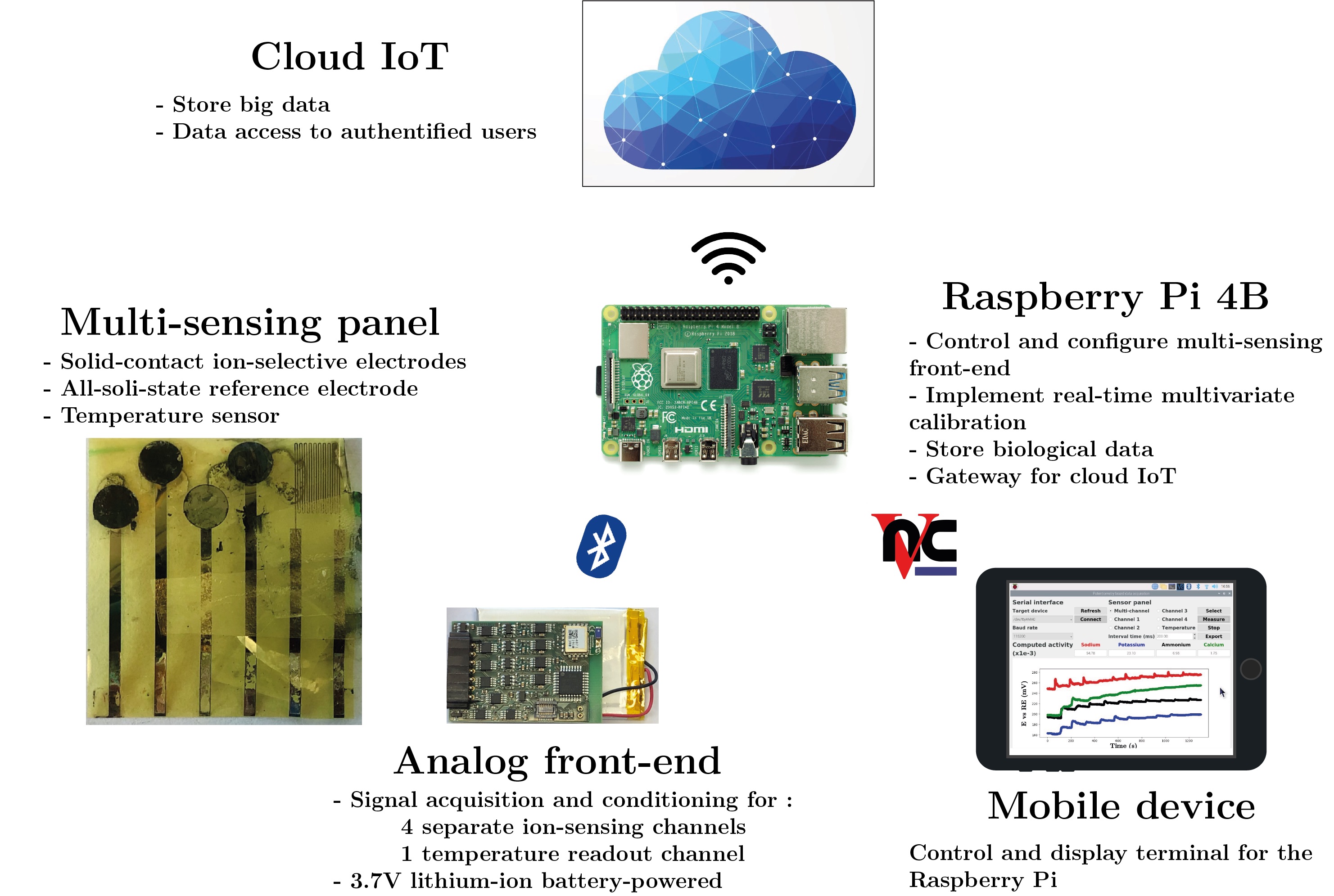
System realization
The proposed approach to increase sensing accuracy is to develop efficient data processing tools and machine learning algorithms in order to cope with the non-linearity introduced by ion interference. A multivariate multi-output support vector regressor is proposed as an accurate, robust, unbiased, low-complexity, and low-latency estimator of the activity of the target ions. An iterative reweighted least-squares procedure is implemented, where the use of a quadratic loss function allows us to build the regression hyperplane by taking into account correlations in the activity of the target ions. Moreover, each iteration has the complexity of an ordinary least-squares minimization. The multivariate regressor is trained with experimental synthetic datasets acquired in artificial sweat samples, and then deployed on a Raspberry Pi, providing a real-time prediction of the activity of sodium, potassium, ammonium, and calcium ions. The edge node serves as gateway to store the measured data on Cloud storage services, in an internet-of-things framework.